
Research
Research Contents
Plant Biophysics/Biochemistry Research Laboratory studies physiological responses in crop plants under various environmental stresses (heat, salt, and water stress conditions) particularly at cell and organelle level. We are also interested in the development of methodologies, focusing on plant water relations.
Development of single-cell metabolomics
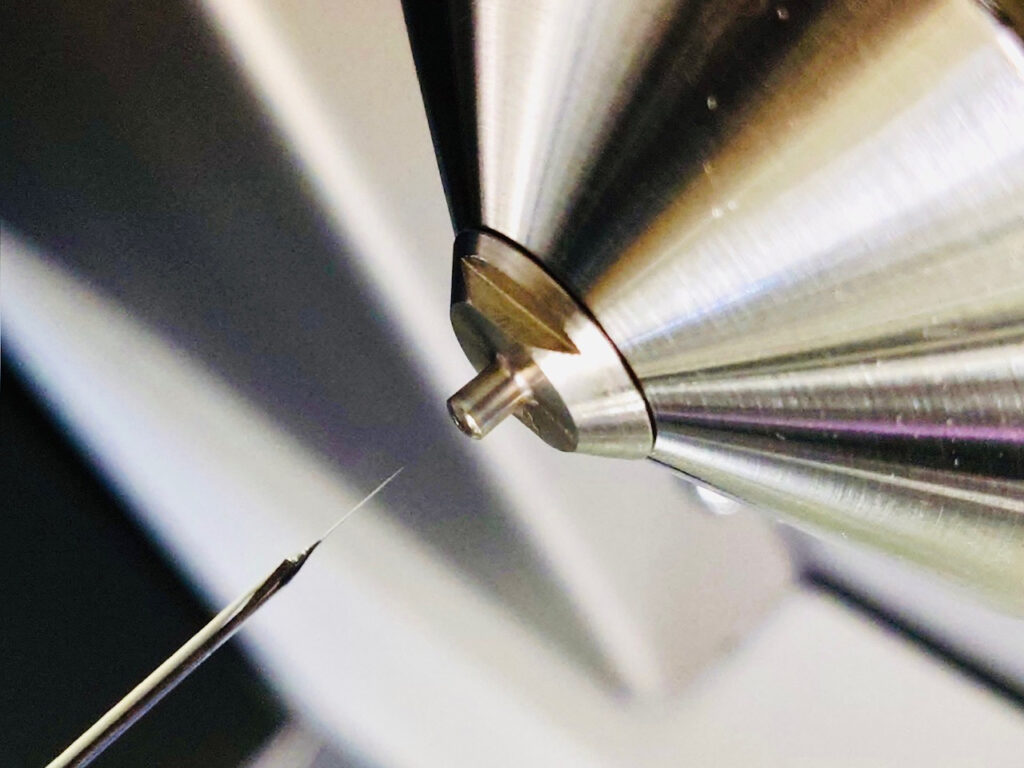
Fig. 1 Single-cell analytical method, called ‘picolitre pressure-probe electrospray-ionization mass spectrometry
(picoPPESI-MS)’ (Nakashima et al., 2016)
By combining a cell pressure probe with Orbitrap mass spectrometer, we have developed a powerful single-cell analytical method, called ‘picolitre pressure-probe electrospray-ionization mass spectrometry (picoPPESI-MS)’(Nakashima et al., 2016).

Fig. 2 The picoPPESI-MS system in the laboratory.
This technique allows us to conduct the various experiments in intact plants and ionize the metabolites in the picoliter cell sap directly collected from the target cells by the means of pressure probe. And subsequently, metabolome analysis can be performed in real-time without the need of any pretreatment prior to the MS analysis (Nakashima et al., 2016). It should be emphasized that this analytical method was designed to be performed in the picoliter fluids collected from the target cells, knowing the physiological status, such as turgor in the ‘living’ cells (Nakashima et al., 2016). This method also allows to determine the volume of sap with a transparent capillary tip, if necessary (Nakata et al., 2022).
In our laboratory, matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI TOF-MS) is also introduced. Similarly, the combinations of pressure probe and MALDI mass spectrometry techniques can be used for studying molecular biology at cell level in intact plants (Gholipour et al., 2010).
Regarding mass spectrometry research, we have been cooperating with Prof. R. Erra-Balsells of the University of Buenos Aires, which has produced three Nobel laureates in Physiology/Medicine and Chemistry related to sugar chemistry and sugar metabolisms and has the academic cooperation agreement with Ehime University. Prof. Dr. Erra-Balsells specializes in mass spectrometry, photochemistry, and sugar chemistry. She visits this laboratory for four months every year, and teaches basic chemistry and mass spectrometry techniques for graduate research study.
We use these single-cell metabolomics to study various physiological disorders in plants, such as heat-induced rice chalky formation (Fig. 3).

Fig. 3 Typical heat-induced chalky rice, called ‘white-back rice’.
We also developed a carbon tracer analysis in plants using Orbitrap mass analyzer using the steady carbon isotope (Wada et al., 2014, 2017). Currently, we are further improving picoPPESI-MS and related analytical methods.
More information
Plant stress responses and cell heterogeneity

Fig. 4 Tip insertion into developing single pollen grains (Wada et al., 2020).
We have been using single-cell metabolomics and various plant water status measurements to investigate mechanisms of various physiological disorders and plant responses under environmental stress conditions (See below).
- Tomato trichomes
- Single-cell metabolite profiling of stalk and glandular cells of intact trichomes with internal electrode capillary pressure probe electrospray ionization mass spectrometry
Nakashima et al. 2016 Analytical Chemistry - Dynamics and stabilization mechanism of mitochondrial cristae morphofunction associated with turgor-driven cardiolipin biosynthesis under salt stress conditions
Nakata et al. 2022 Scientific Reports
- Single-cell metabolite profiling of stalk and glandular cells of intact trichomes with internal electrode capillary pressure probe electrospray ionization mass spectrometry
- Rice endosperms under heat/foehn-induced dry wind conditions
- Increased ring-shaped chalkiness and osmotic adjustment when growing rice grains under foehn-induced dry wind condition
Wada et al. (2011) Crop Science - Rice chalky ring formation caused by temporal reduction in starch biosynthesis during osmotic adjustment under foehn-induced dry wind.
Wada et al. (2014) PLoS ONE - Evidence for preservation of vacuolar compartments during foehn-induced chalky ring formation of Oryza sativa L.
Hatakeyama et al. (2018) Planta - Multiple strategies for heat adaptation to prevent chalkiness in the rice endosperm
Wada et al. (2018) Journal of Experimental Botany
Endosperm cell size reduction caused by osmotic adjustment during nighttime warming in rice
Wada et al. (2021) Scientific Reports - Metabolic coordination of rice seed development to nighttime warming: In-situ determination of cellular redox states using picolitre pressure-probe electrospray-ionization mass spectrometry
Chang et al. (2021) Environmental and Experimental Botany
- Increased ring-shaped chalkiness and osmotic adjustment when growing rice grains under foehn-induced dry wind condition
- Rice pollen metabolisms
- On-site single pollen metabolomics reveals varietal differences in phosphatidylinositol synthesis under heat stress conditions in rice
Wada et al. (2020) Scientific Reports
- On-site single pollen metabolomics reveals varietal differences in phosphatidylinositol synthesis under heat stress conditions in rice
- ‘Roly-poly toy’ motion during pollen exudation
- ‘Roly-poly toy’ motion during pollen exudation promotes rapid pollen adhesion in rice
- Wada et al., (2025) Communications Biology
- Lignin biosynthesis in Norway Spruce
- Ray parenchymal cells contribute to lignification of tracheids in developing xylem of Norway Spruce
Blokhina et al. (2019) Plant Physiology (journal cover)
- Ray parenchymal cells contribute to lignification of tracheids in developing xylem of Norway Spruce
- Apple watercore formation
- Direct evidence for dynamics of cell heterogeneity in watercored apples: turgor-associated metabolic modifications and within-fruit water potential gradient unveiled by single-cell analyses
Wada et al. (2021) Horticulture Research
- Direct evidence for dynamics of cell heterogeneity in watercored apples: turgor-associated metabolic modifications and within-fruit water potential gradient unveiled by single-cell analyses
More information
Plant water status measurements


Fig. 5 Satsuma mandarin orchards in Maana area, Ehime Prefecture and the water status measurement using a pressure chamber.
In our laboratory, cell pressure probes, isopiestic psychrometers, pressure chambers, and nanolitre osmometer are used to measure the water status of plants, i.e., water potential, osmotic potential, and cell turgor in plants. We are also working on the water status measurements under field conditions from the practical perspective.
References
Boyer(1995)Measuring the water status of plants and soils, Academic Press
Steudle (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue and organ level. 5-36, BIOS Scientific Publishers
More information